
Hasan Hazim Alsararatee
Acute Medicine/ SDEC, Northampton General Hospital NHS Trust, Northampton, Northamptonshire, UK.
*Corresponding Author: Hasan Hazim Alsararatee, Acute Medicine/ SDEC, Northampton General Hospital NHS Trust, Northampton, Northamptonshire, UK.
Received Date: January 30, 2024
Accepted Date: February 12, 2024
Published Date: February 15, 2024
Citation: Hasan Hazim Alsararatee. (2024) “Atypical Presentation of Pneumonia Mimicking Pulmonary Embolism.”, International Journal of Medical Case Reports and Medical Research, 2(2); DOI: 10.61148/2994-6905/IJMCRMR/029.
Copyright: © 2024. Hasan Hazim Alsararatee. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Summary:
This case report highlights the diagnostic challenge arises from the varied clinical presentation, complicating the differentiation between infarction pneumonia, community-acquired pneumonia (CAP), and pulmonary embolism (PE). The author reports a female patient in her late seventies who presents with worsening right-sided pleuritic chest pain, exertional dyspnoea, and productive cough. She was initially treated for CAP with oral amoxicillin 500 mg QDS and clarithromycin 500 mg twice daily for 5 days. She denied any night sweats or weight loss. On lung auscultation, she had right lower lobe crepitations. Subsequently, she underwent a computed tomography pulmonary angiography (CTPA) to rule out PE which demonstrated filling defects in the right pulmonary artery and right lower lobe patchy consolidation. The patient was treated with rivaroxaban 15 mg BD for 21 days followed by 20 mg OD for 4 months, and also treated for CAP with ceftriaxone 2 g OD for 7 days.
Background:
PE is characterized by the obstruction of the pulmonary artery or its branches, typically caused by a thrombus originating from the lower extremity veins [1]. Patients might present with exertional dyspnoea, tachycardia, shortness of breath, chest pain and syncope [2]. Thus, it requires prompt and accurate diagnosis. CTPA is often the preferred diagnostic method due to its high sensitivity and specificity [3]. However, in cases where CTPA is contraindicated, a ventilation-perfusion (V/Q) scan may be conducted, despite its lower sensitivity and specificity [4]. Distinguishing between infarction pneumonia, CAP and PE in clinical practice encounter significant challenges particularly when symptoms overlap. Infarction pneumonia can occur after PE which can mimic the diagnosis of PE. Furthermore, it can develop to a CAP as a result of the necrotic lung tissue which can mask the PE. More importantly, PE can be provoked by CAP due to hypercoagulability state, pro-inflammatory and endothelial damage [5]. The diagnosis of pneumonia can be established in emergency settings based on patients’ symptoms and high inflammatory markers with consolidations on CXR. However, identifying pneumonia from PE is extremely challenges if CTPA is not performed. Recognizing these complexities is essential for timely intervention and effective management of patients presenting with symptoms suggestive of both pneumonia and PE.
Case Presentation:
A female patient in her late seventies was referred from General Practitioner (GP) to Same Day Emergency Care (SDEC) with right-sided pleuritic chest pain, productive cough, and exertional dyspnoea for the last one week. She has a background of hypertension manages on Ramipril 5 mg once daily. She denies any significant family history of respiratory or cardiovascular diseases. Her symptoms initially manifested as a dry cough; her GP initiated treatment for CAP with oral amoxicillin 500 mg four times daily. However, as the cough progressed to productive and mild right-sided chest pain, clarithromycin 500 mg BD for 5 days was added. However, due to persisting symptoms, a referral to SDEC was made for further evaluation.
During the examination, blood pressure measured 132/80 mmHg, and the heart rate was 75 beats per minute. Right lower base crepitations were detected upon lung auscultation. The Wells score indicated a moderate risk [3], alongside with a D-dimer level of 2210 ng/m. This prompted a CTPA investigation due to ongoing symptoms, heightening suspicion for an undiagnosed PE.
Investigations:
A chest x-ray was obtained, revealing right sided blunting costophrenic angle which may represent infection/pleural effusion (Figure 1). The electrocardiogram (ECG) showed a normal sinus rhythm (Figure 2). Blood tests, including C-reactive protein (CRP) which is 187 mg/L 0 - 5 and white blood cell count (WBC): 16.3 109/L 4.0 - 10.0, despite having oral amoxicillin 500 mg QDS by the GP for 7 days. Troponin T <13 ng/L. Urea and Electrolytes were normal. The Dimmer test result was 2210 ng/m, indicating high levels. Additionally, a COVID-19 test was conducted, which yielded negative results. Blood cultures and sputum gram stain with culture were negative, as were urinary antigen tests for Streptococcus pneumoniae and Legionella. N-terminal pro B-type natriuretic peptide (NT-pro BNP) and Echocardiogram was normal left ventricular (LV) function and no wall motion abnormalities with normal right ventricular (RV) function size.
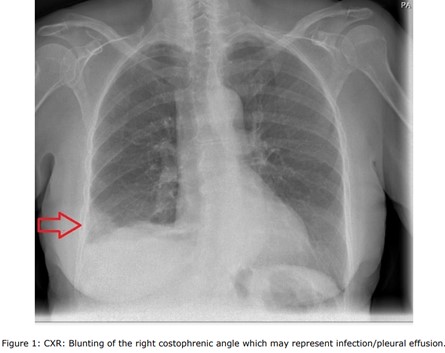
Figure 1: CXR: Blunting of the right costophrenic angle which may represent infection/pleural effusion.
Figure 2: 12 leads ECG: Normal Sinus Rhythm.
Given the persistent patient's symptoms despite completing the course of oral antibiotics, and raising D-dimer, a CTPA was performed to rule out PE which demonstrated multiple filling defects in the right lower pulmonary artery and its segmental with subsegmental branches, and patchy consolidations in the right lower lobe with mild right pleural effusion suggestive of infective/inflammatory aetiology ( Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
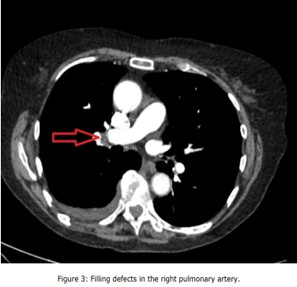
Figure 3: Filling defects in the right pulmonary artery.

Figure 4: Filling defects in the right pulmonary segmental branches.

Figure 5: Filling defects in the right pulmonary subsegmental branches
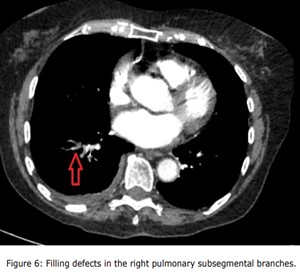
Figure 6: Filling defects in the right pulmonary subsegmental branches

Figure 7: CTPA lung window shows patchy consolidations in the right lower lobe with mild right pleural effusion suggestive of infective/inflammatory aetiology.
Treatment:
As the PESI score was low risk and the patient is clinically stable. She was discharged with rivaroxaban 15 mg BD for 21 days followed by 20 mg OD for four months [6]. Furthermore, she was treated for CAP with IV ceftriaxone 2 g once daily for 7 days.
Outcome and follow up:
After 7 days of having the above antibiotics, CRP inflammatory markers have improved, and the patient felt well with no chest pain or cough and the breathless is improving. She is doing well on follow up.
Discussion:
Pulmonary embolism (PE) is a common cardiology emergency characterized by the obstruction of a pulmonary vessel due to a dislodged blood clot, typically caused by a thrombus originating from the lower extremity veins which can be acute life threatening. Clinical manifestations include symptoms such as shortness of breath, pleuritic chest pain, coughing, orthopnoea, painful swelling in the calf or thigh, wheezing, haemoptysis, and, less frequently, heart arrhythmia [7]. In more severe cases, it may present with a syncopal attack or, more critically, as circulatory collapse [8]. PE can be diagnosed on CTPA due to its high sensitivity and specificity, revealing a filling defect upon contrast enhancement in the pulmonary artery or any of its branches [9]. However, if CTPA is contraindicated, a ventilation-perfusion (V/Q) scan can be performed. This alternative method shows impaired perfusion with normal ventilation, but it has low sensitivity and specificity [10].
Distinguishing between pneumonia and PE in clinical practice presents significant challenges, potentially resulting in diagnostic delays and subsequent complications, culminating in delayed treatments and serious implications [11]. This complexity is accentuated by various situations, such as infarction pneumonia that happen after PE and the simultaneous occurrence of community-acquired pneumonia (CAP) with underlying PE. In this case study, the PE progressed to pulmonary infarction, leading to infection of the necrotic lung tissue [12]. The rarity of pulmonary infarction following PE is attributed to the dual blood supply of the lung, occurring at a rate of 10% [13]. Recent literature indicates that elderly patients with cardiopulmonary disease are more prone to experiencing secondary pulmonary infarction following a PE because of the poor collateral circulation [14]. Several studies have observed a higher prevalence of infarction in the right lung [15 ,16]. This observation is consistent with the findings in this particular case report. However, the underlying reason for this phenomenon remains unknown.
A heightened index of suspicion is crucial for the early diagnosis of PE and pulmonary infarction, especially in elderly patients with PE risk factors who present symptoms such as cough, pleuritic chest pain, fever, and shortness of breath, along with consolidation on CXR. In such instances, prompt consideration of a CTPA scan is essential to confirm or exclude PE. In this case report, despite the patient receiving a course of oral antibiotics, the sustained consolidation on CXR raises a high suspicion of PE.
The concurrent presence of CAP and PE represents another manifestation of the link between pneumonia and PE [17]. This situation is infrequent and poses a diagnostic challenge due to significant overlap in clinical symptoms, including fever, cough, shortness of breath, and leucocytosis. In a study, chest pain, shortness of breath, haemoptysis, and fever were identified as independent risk factors for PE in patients initially diagnosed with pneumonia [18]. Conversely, emphasis on dyspnoea and/or chest pain in PE, contrasting with fever, chills, and/or cough, was noted in another study [19]. Notably, only 3.3% of patients initially diagnosed with pneumonia in the latter study were later identified as having acute PE. In our case, initial suspicion leaned towards atypical pneumonia; however, the subsequent lack of therapeutic response and additional diagnostics prompted a reconsideration of the diagnosis.
CAP may increase the risk for venous thromboembolism, possibly through a pro-inflammatory, hypercoagulable state contributing to PE development. CAP can mimic PE, leading to delayed diagnosis and treatment [20]. Hence, clinical indicators such as elevated D-dimer levels, haemoptysis, and sudden deterioration of respiratory function or chest pain should prompt suspicion of concomitant PE. In our case report initially, the patient presented with fever, productive cough, right-sided pleuritic chest pain, and severe shortness of breath, the diagnosis of PE was less likely due to the dominance symptoms that suggest a chest infection. However, clinical parameters, including haemoptysis, and worsening right sided chest pain with high D. dimer after the initial improvement, directed the suspicious to concealed PE.
The following four cases evident the diagnostic challenge of differentiating between pneumonia and PE as summaries in Table 1.
The first case involved a patient initially suspected of atypical pneumonia, with symptoms such as cough and fever. Despite initial treatment, further evaluation revealed a massive right-sided PE, highlighting the potential mimicry of pneumonia by PE. The second case, the patient presented with a sudden onset of exertional dyspnoea, a symptom commonly associated with pneumonia. However, subsequent diagnostic imaging disclosed bilateral segmental PE, emphasizing the deceptive similarity in clinical presentation. The third case, presenting with a productive cough, pleuritic chest pain, and haemoptysis, initially led to a pneumonia diagnosis. However, unexpected findings of intraluminal thrombus in the pulmonary arteries during a contrast-enhanced CT chest shifted the diagnosis towards PE. Fourth case involved a patient admitted for degenerative spinal disease, exhibiting fever and breathlessness. Initially treated for community-acquired pneumonia, the persistence of symptoms and subsequent diagnostic imaging, including a CTPA, revealed features consistent with PE, underscoring the challenge of distinguishing between pneumonia and PE. These cases collectively highlight the importance of a nuanced approach to diagnosis when faced with pneumonia-like symptoms, emphasizing the need for a comprehensive evaluation to discern potential underlying pulmonary embolism.
Given the clinical presentation, we propose pneumonia as a provoked risk factor for developing PE in our case report. Recognizing the presence of underlying PE when diagnosing pneumonia holds therapeutic significance, as it necessitates initiation of anticoagulation therapy. Consequently, it is crucial to assess the patients comprehensively and requesting appropriate investigations accordingly to exclude or confirm PE. In conclusion, it is imperative to acknowledge the association between pneumonia and PE and carefully assess and evaluate the clinical and laboratory findings, as well as CXR, when deciding to conduct a CTPA scan for patients developing pneumonia, aiming to promptly identify concealed PE.
Learning points/ Take home messages.
|
Case |
Authors |
Location |
Age |
Initial presentations and durations |
Comorbiditie s and risk factors for PE. |
Radiographic features pre-CTPA and post CTPA |
Suspicious for CTPA and findings |
Treatment and follow up. |
|
1 |
Mohammed et al., (2022) (5) q |
Qatar |
35 years old male |
Four days history Productive cough, fever, shortness of breath right sided pleural effusion. |
Not known to have Past medical history, no risk factors for PE |
CXR: Right sided pleural effusion (? parapneumonic effusion). Non enhance CT chest shows right sided consolidation with bilateral lower lobe atelectasis changes. |
Two days after starting the patient on Levofloxacin 750 mg OD, the fever has resolved but after two weeks follow up he remained breathless and right sided chest pain and haemoptysis. O/E: reduced air entry to the right lower base. Thus, CTPA was conducted which shows massive right sided PE with RV dilation. |
The patient, initially managed with Enoxaparin at 1.5 mg per kg, was discharged in a stable condition and is now thriving on Rivaroxaban, as evidenced by their positive status during the two-week follow-up in the clinic. |
|
2 |
Beckman et al., (2020) (21) |
Sweden |
51 years old male |
Udden onset of exertional dyspnoea within 4-5 weeks, worsening the past 48 hours. |
Not known to have Past medical history, no risk factors for PE |
Prior to the CTPA, unknown radiological features were present, but with inflammatory markers not significantly elevated and an incomplete RBBB on the ECG suspicion of PE emerged. |
The CTPA revealed bilateral segmental pulmonary embolism with right ventricular dilation. |
Admitted to the hospital for two days, the patient received subcutaneous tinzaparin at 18,000 units daily. Upon discharge, the patient was prescribed apixaban at 5 mg twice daily, with a recommended treatment duration of 6 months. He is doing well on regular follow ups. |
|
3 |
Sooraj et al., (2020) (18) |
India |
75 years old male |
presented with a one-week history of productive cough, pleuritic chest pain, and haemoptysis, accompanied by a SpO2 of 88% on room air, which improved to 94% with 4L O2. On examination, bilateral crepitations were noted in the intrascapular areas. The ECG revealed sinus tachycardia. |
Not known comorbiditie s |
a CXR demonstrated a mass lesion in the right paracardiac region. The patient was admitted and treated for pneumonia with intravenous antibiotics, steroids, and oxygen but a contrast- enhanced CT chest for confirmation of the pneumonia diagnosis was performed. |
PE was not suspected but the CT findings were intraluminal thrombus in the right and left pulmonary arteries distal to the upper lobar branches and extension of thrombi into segmental branches with consolidation of medial and lateral segments of the right middle lobe and basal segments of the left lower lobe with RV strain pattern. |
Thrombectomy was not suitable option for the patient. Consequently, the patient received intravenous streptokinase at a dosage of 2.5 lakh IU over a 30-minute interval. Subsequent to this intervention, significant improvement transpired over the following 6 days, culminating in the patient's discharge with continued anticoagulation. |
|
4 |
Alvin et al., (2019) (22) |
Malaysia |
A 70- year-old man |
Admitted for eight days due to degenerative spinal disease and prolapsed intervertebral discs affecting L3/L4, L4/L5, and L5/S1, the patient presented with breathlessness, fever, and a Spo2 of 90%. Coarse crackles were auscultated over the right lower zone. |
PMH: hypertension , diabetes mellitus, dyslipidaemi a and ischemic heart disease |
CXR revealed consolidation in the right lower lobe. He was treated for CAP with Ceftriaxone 2 g OD and required 10 L of oxygen, but he was still breathless, tachypnoeic. ABG show Type one respiratory failure. Thus, the suspicious of PE was raised. DVT scan was negative. |
CTPA demonstrated filling over the secondary branch of the descending right pulmonary artery without RV dilation and right lower lobe consolidation. |
Fondaparinux 10 mg once daily and oral warfarin were initiated after the patient's condition stabilized. Once the target INR of 2 to 3 was achieved, the subcutaneous Fondaparinux was discontinued. The plan was to continue oral warfarin for 3 months, with unknown clinical follow-up. |
Patients’ perspective:
I am so thankful for the attentive care and the discovery of my pulmonary embolism (PE) through the CT scan. I genuinely thankful for the dedicated medical teams and the author for their commitment to my well-being. I have received excellent care with clear follow up.